|
BY NIKOLA STIKOV
At the OHBM 2015 Annual Meeting in Honolulu, HI, the OHBM Communications Committee (or ComCom as we like to call it) was created by the OHBM Council. They appointed Randy Gollub as its first Chair and Niko Kriegeskorte as Chair-Elect. Over the fall and winter Randy and Niko worked with OHBM staff to recruit volunteers and create a structure for this new and exciting initiative. The first step after assembling a group of eager and extremely talented Committee members was to divide the large group into four different specialized teams and a quarter of the members were assigned to the ‘Member Communication’ team. Our task was daunting. How do you communicate year-round with a membership that spans so many disciplines, continents and backgrounds? Do you create separate newsletters, Facebook pages and YouTube channels? Do you hold monthly meetings in which you assign specific tasks targeting specific audiences? Or do you put all this under one umbrella, call it a blog, and let the OHBM members decide what to do with it? If you are reading this, then the answer is obvious. We began with a blog because it was the most inclusive (and easiest!) platform we could think of, and then allowed everything else to evolve organically. The “Member Communication” Team became the “Blog Team” and over the past year we have published 51 blog posts, featuring over 40 contributors from 11 countries. The blog has received over 165,000 page visits from 63,000 unique visitors, we’ve created 12 videos for our YouTube channel and OHBM had over 345,000 impressions on Twitter. The OHBM Communication Committee’s work has also been featured in the Huffington Post and cited by the New York Times. Not bad for a one-year old...
0 Comments
 Figure 1. Karla Miller adorned with a student’s research. Figure 1. Karla Miller adorned with a student’s research. BY GUEST AUTHOR ERIKA RAVEN This post originally appeared on the ISMRM blog and in the MRM Highlights magazine. Republished (and slightly modified) with permission. Karla Miller is a professor of biomedical engineering at the Oxford Center for Functional MRI of the Brain (FMRIB, pronounced “fim-rib” for short). She directs the FMRIB Neuroscience Physics group, which specializes in many projects, from pulse sequence development to biophysical tissue modeling. More recently, she’s been a key figure of the UK Biobank, a mega-sized data initiative charged with imaging 100,000 adults by 2022. Karla is also a plenary speaker at the upcoming OHBM meeting in Vancouver, education chair of this year’s ISMRM meeting in Honolulu, and is poised to chair the entire ISMRM program for the 2018 meeting in Paris. In our interview, Karla makes connections between the many themes in her life, which ultimately are resolved by finding the right balance. SHRUTI GOPAL VIJ
COBIDAS: OHBM Committee on Best Practice in Data Analysis and Sharing Neuroimaging researchers study both the structural and functional organization of the brain in health as well as a variety of neuropsychological conditions. Extensive exploration has been conducted over the past few decades using novel experimental and analysis techniques. However, recent evaluations of these techniques have highlighted concerns that published scientific results are less reliable or reproducible primarily due to the lack of transparency in research practices. To address the need for outlining principles of scientific research that will increase transparency and reproducibility in human neuroimaging, the Organization for Human Brain Mapping created the Committee on Best Practices in Data Analysis and Sharing (COBIDAS). COBIDAS has initiated efforts in distilling best practices for open science in human neuroimaging and has compiled suggestions for specific research practices to support open data and open methodology. These suggestions were composed in a report that was drafted and ratified using community collaboration. While the report itself provides a detailed description of best practices as well as approaches to avoid, a commentary that reviewed the impact of COBIDAS and the challenges ahead was published in February 2017. The commentary underlines the importance of reproducibility and highlights the different aspects of replicating a study including generalizability over methods, materials and results as shown in Figure 1. One practice that was universally recommended is the transparent and complete reporting of all facets of a study, allowing the critical reader to evaluate the work and fully understand its strengths and limitations. Additionally, thorough reporting will equip the reader/other researchers with a detailed knowledge of how to fully replicate the study. COBIDAS MRI report presents reporting checklists in Appendix D that researchers can follow while preparing manuscripts. These checklists are highly comprehensive and run the gamut of stages a human neuroimaging study passes through. The report also pinpoints specific bad practices, especially in statistical modeling and inference (Pages 11 & 12), and provides concrete recommendations for avoiding them. COBIDAS also suggest that researchers at all levels be mindful of these considerations while conducting individual studies and compiling manuscripts. BY NILS MUHLERT Every year OHBM receives thousands of abstracts, each a snapshot of the intensive work of individuals or teams of scientists. Contained within this bumper crop of science are telling replication studies, novel experimental designs, methodological advances and fresh insight into the workings of the human brain. This harvest, whilst welcome, necessitates difficult decisions about which prize specimens to highlight as talks, and which to promote through posters. For those of us yet to work on program committees this process of selection can seem opaque - we can perhaps predict the established trends and exciting developments from year to year, but how do committees decide which emerging fields will pique the interests of the majority of OHBM attendees? Here we find out the decisions that were made in deciding on the OHBM 2017 program, through discussion with the Program Chair and Stanford Neurologist, Mike Greicius.  Figure 1. Mike Greicius, OHBM Program Chair 2016-17 Figure 1. Mike Greicius, OHBM Program Chair 2016-17 Nils Muhlert (NM): First, can you tell us about your career path into Neurology and Neuroimaging? Mike Greicius (MG): I was a French major as an undergraduate but was able to get my pre-med classes done at the same time. I went to Columbia for medical school but was allowed to defer my matriculation for 1 year so that I could "teach English" in Prague and Paris, which, in fact, I sort of did. Taking a year off allowed me to hit medical school refreshed. It was as a second-year medical student at Columbia that I had my epiphany about neuroscience. I was in a lecture on aphasia and the professor, Richard Mayeux (now the chair of Neurology at Columbia), had a videotape showing a conversation with one of his Wernicke's aphasia patients. I was thunderstruck and knew, from that point, that I wanted to be a behavioral neurologist. In my head I summed it up as something like "why study the kidney or the liver when you can study the one organ that talks back to you?". I did my one-year of internal medicine at Columbia and then residency training in Neurology at Harvard, which is a fondly remembered blur. Fondly, mainly because it is blurry. At the time I was rather zombie-like, overworked, underpaid, etc. Those were three tough but formative years. In 2000, on finishing my residency I came to Stanford for an fMRI fellowship (with Allan Reiss and Vinod Menon) which I combined with a behavioral neurology fellowship (done at UCSF with Bruce Miller). The sun came out and everything changed. I was delighted to learn that my wife still loved me and that I could now spend time awake with my then 2-year old son. BY THE OHBM STUDENT AND POSTDOC SPECIAL INTEREST GROUP
OHBM is open to brain mappers of all ages and career stages, and the students and postdocs are a vital part of the society. Despite strong representation by PIs, there remains a need for trainees to have their own platform where they could discuss and disseminate information specifically relevant to them, such as job opportunities, funding, scholarships, and awards. The OHBM Student and Postdoc Special Interest Group (SIG) was set up to achieve these aims, while also creating the opportunity to interact in-person during the famous Monday Night Social at the annual OHBM meeting. In 2017, however, there is more to the SIG’s efforts than the above mentioned activities. The SIG has a new initiative on mentorship to help prepare trainees to transition to early-career researchers; that includes a symposium and the launch of an online international mentoring forum. The symposium is slated to include a panel discussion, in addition to talks by academics that will help provide career direction to human brain mapping postdocs and students. The new online mentoring forum will pair students, postdocs, and researchers in online mentoring relationships that cross the globe; these mentoring pairs will have the opportunity to meet in person during the annual meeting. Together, these initiatives offer information about possible career paths, and organize information seekers and givers into a cohesive unit. Such information will breed confidence in early-career researchers and help them become the leaders of future research initiatives. To do all this and more, we have put together a team of excellent students and postdocs to lead the SIG. These committee members come from varied backgrounds in their research, and are representative of the diversity of our community at-large. 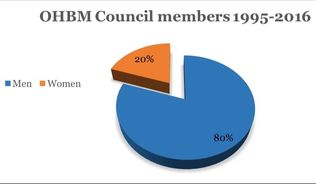 BY THE OHBM DIVERSITY AND GENDER TASK FORCE It’s often been said that the best predictor of future history is past history. Thus, after comments at the OHBM town hall meeting in Geneva regarding the current gender imbalance of Council members (1 female, 14 males), the Council made a decision that something needed to be done to enhance gender equity and geographic diversity. Thus, the OHBM Diversity and Gender Task Force was formed. If it is true that the best predictor of future history is the past, then it was important for the committee to obtain a historical perspective of how OHBM has been doing with respect to women in leadership roles. Perhaps the current 14:1 relationship between males and females is merely a dip in what was otherwise a balance in gender. Thus, we took a close look at the distribution of gender within leadership roles and education at the OHBM annual meetings. Like all good scientists, we will let the data speak for itself (See figures below). Of course, like all good scientists, we also like to say ‘in brief…’ the ratio between males to females in leadership positions, awards, and keynote presentations is about 5 to 1 over the history of the organization. The one area in which there appears to be a transition to greater gender equality is in the Keynote lectures. However, such a transition has not been present in Council positions, which was highlighted at the Geneva Town Hall Meeting. The gender distribution of the general membership is not known because this information has not been requested in the past. Similarly, race and ethnicity of the membership is also not known. However, the gender distribution of poster submissions is approximately 50:50. |
BLOG HOME
Archives
January 2024
|
 RSS Feed
RSS Feed